CAPRI Clinical Trials
Below is a list of clinical trials run by the CAPRI group.
To obtain a protocol or open a trial at your centre, please use the contact information below.
To obtain a protocol or open a trial at your centre, please use the contact information below.
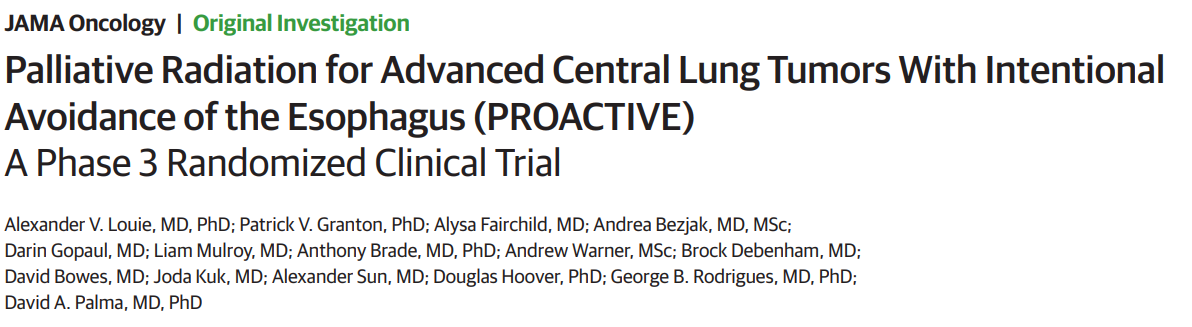
PROACTIVE
A randomized phase III study of Palliative Radiation of Advanced Central Tumors with Intentional avoidance of the Esophagus
|
Study Question:
Can esophageal sparing improve quality of life for patients receiving palliative radiation for non-small cell lung cancer? Principal Investigators: Dr. Alexander Louie (Radiation Oncology) Dr. Patrick Granton (Radiation Physics) Target Accrual: 90 patients (45 patients in each arm) Current Accrual: 90/90 patients Current Status: Published in JAMA Oncology |
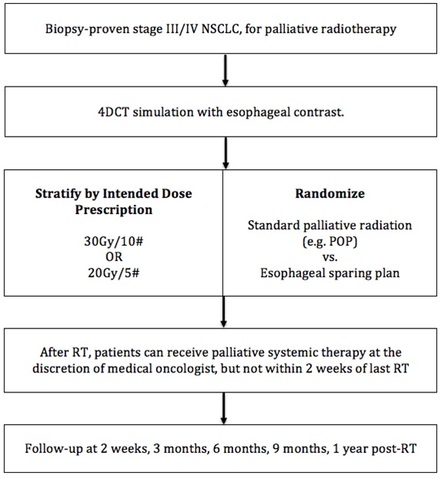
PET-BOOST
A randomized phase II trial to assess the efficacy and safety of selective metabolically adaptive radiation dose escalation in locally advanced non-small cell lung cancer receiving definitive chemoradiotherapy
|
Study Question:
In stage III non-small cell lung cancer patients receiving chemoradiation, dose dose escalation to metabolically active tumor volumes improve locoregional control and overall survival? Principal Investigators: Dr. Alex Sun (Radiation Oncology) Dr. Jean-Pierre Bissonnette (Radiation Physics) Target Accrual: 74 patients (37 patients in each arm) Current Accrual: 74/74 patients Current Status: Data Maturing, Analysis expected late 2023 ClinicalTrials.gov Identifier: NCT02788461 |
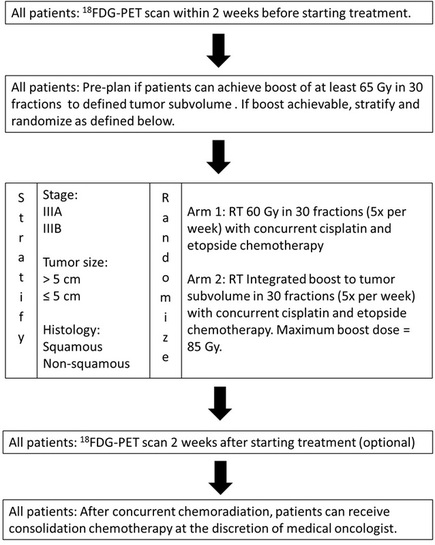
STOP
Stereotactic radiotherapy for oligo-progressive cancers: A randomized phase II trial
|
Study Question:
What is the impact of stereotactic radiation in patients with metastatic non-small cell lung cancer who have oligo- progressive disease while on systemic therapy? Principal Investigators: Dr. Devin Schellenberg (Radiation Oncology) Dr. Patrick Cheung (Radiation Oncology) Target Accrual: 90 patients Current Accrual: 90/90 patients Current Status: Reporting in late 2023 (ASTRO annual meeting) ClinicalTrials.gov Identifier: NCT02756793 |
SUNSET
Stereotactic body radiotherapy for ultra-central non-small cell lung cancer: A safety and efficacy trial
|
Study Question:
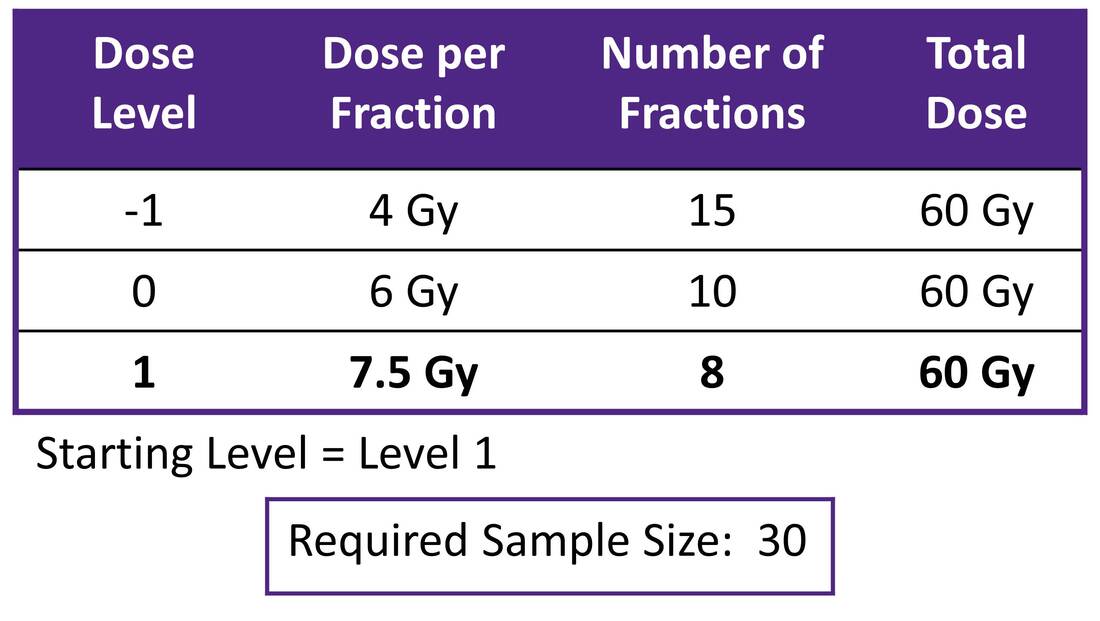
What is the maximally tolerated dose of radiotherapy for ultracentral non-small cell lung carcinoma associated with a 30% or lower rate of grade 3-5 toxicity occurring within 2 years of treatment? Principal Investigator: Dr. Meredith Giuliani (Radiation Oncology) Target Accrual: 30 patients Current Accrual: 30/30 patients Current Status: Reporting in late 2023 ClinicalTrials.gov Identifier: NCT03306680 |
ASPIRE-ILD
Assessment of precision irradiation in early non-small cell lung cancer and interstitial lung disease: A phase II trial
|
Study Question:
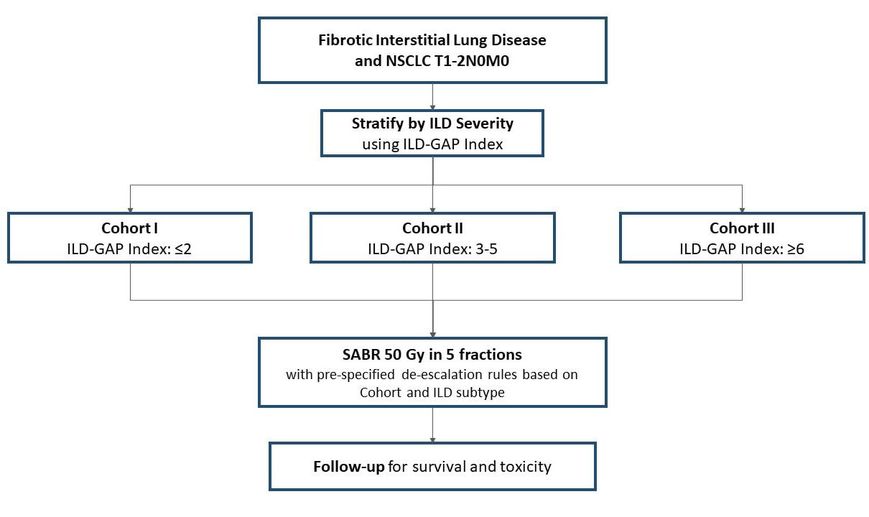
What are the survival and toxicity outcomes associated with the use of SABR in patients with ILD? Principal Investigators: Dr. David Palma (Radiation Oncology) Dr. Alexander Louie (Radiation Oncology) Dr. Chris Ryerson (Respirology) Target Accrual: 39 patients Current Accrual: 39/39 patients Current Status: Reporting in late 2023 (ASTRO annual meeting) ClinicalTrials.gov Identifier: NCT03485378 |
Selection of Trials for CAPRI
Trial proposals are solicited from investigators across Canada. All trials are peer-reviewed in a double-blind process by both Canadian and International experts. Proposals are scored based on novelty, scientific relevance, feasibility, methodology, and potential for clinical impact. If you are an investigator wishing to propose a trial, please contact us below.
Funding of CAPRI Trials
CAPRI trials are funded through philanthropic donations made to the London Health Sciences Foundation, and operating research funds from the Ontario Institute for Cancer Research.